Huron IRB - Reportable New Information (RNI)
Estimated Reading Time: 4 Minutes*Please note a terminology change: this was formerly called an Event Report (ER).
Prior to the Submission
Prior to submitting your RNI in Huron, you should first complete the HRP-216-Template: RNI Supplemental Information Form. This editable Word document can be found in Huron Library [IRB > Library > Templates]. The same form can be used if reporting an event affecting multiple studies. This form should be completed and uploaded as supporting information for every RNI. Importantly, the form captures the corrective action plan.
Navigation
- Make sure you are on the IRB tab.
- Select “Submissions” in the sub-navigator to see your IRB submissions.
- Select the “Active” tab.
- Open the study with the RNI.
.png)
Study Workspace
- Once inside the study workspace, you will select “Report New Information” to open the RNI SmartForm.
.png)
RNI SmartForm
Reportable Information
- RNI short title: Select a brief title for this RNI submission that distinguishes it from other submissions. You can use any unique title shorter than 50 characters.
The short title identifies the RNI throughout the IRB system, such as in your inbox and in the IRB’s list of submissions to review. Format the short title to list the general RNI category followed by a brief description in parenthesis.
For example:- Protocol deviation (missed blood collection)
- Consent issue (wrong version of consent used)
- Harm (participant suffered ischemic stroke)
- Breach of confidentiality (data emailed to unauthorized person)
- Data you became aware of the information: Use the date selector to indicate when you became aware of the information. Please note that this is not the date of form submission, nor the date the reporting event took place.
.png)
- Identify the categories that represent the new information (check all that apply). There are multiple categories in this section. Read through each category and select all that apply to your new information report.
.png)
- Briefly Describe the New Information. Describe in detail the new information being reported. Use complete sentences to describe the information and specify what occurred, where it occurred, and how the event fits into one or more categories of the categories in question three. Indicate if the reportable new information was expected or if this was unexpected; if the RNI was related or unrelated to the research; if any increased risk was involved; and the outcome of the event. Please also describe any corrective actions the study team has already taken to address the issue.
For medical events: include diagnosis, assigned intervention, dose levels and frequency of intervention, relevant history including preexisting medical conditions, diagnostic test results, laboratory assessments, concomitant medications, and relevant dates. Do not include participants’ personally identifiable information.
.png)
- Does this information indicate a new or increased risk, or safety issue? Select Yes if the information indicates an increase in the frequency or magnitude of a previously known risk or uncovers a new risk or safety issue.
- Does the study need revision? Indicate whether or not the study needs revision. If revisions are required, describe them in question four and submit a study modification for review.
- Does the consent need revision? If revisions are required to the consent form, describe them in question four and submit a study modification for review.
.png)
- Related Studies and Modifications: Your study will already be populated, and you can use the ellipsis […] to attach related studies and modifications.
The PI, PI proxies, and primary contact of each related submission are notified at several points in the RNI workflow. In addition, the PI and PI proxies of each related submission can edit the RNI and submit it for review, along with submitting a response to a clarification request. - Attach supporting information. Include a corrective action plan, as applicable. Use this entry field to upload your completed RNI Supplemental Form (HRP -216), which includes any corrective action plans or any additional information relevant to the RNI.
.png)
Final Page
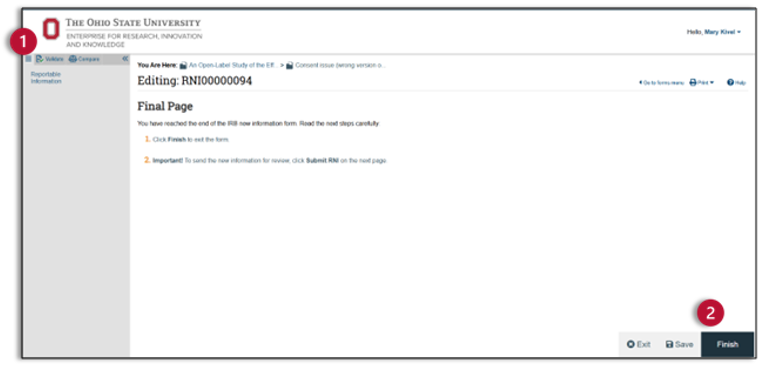
- After you have completed the RNI SmartForm, you will come to the Final Page. You can check your submission for errors using the validate and compare features at the top of the left navigator.
- After you have corrected any errors, you are now ready to finish the RNI SmartForm. You will notice that the RNI SmartForm now has a finish button. Click finish and exit the SmartForm.
RNI Workspace
Before entering the RNI in the RNI SmartForm, you were in the Study Workspace. After completing the RNI SmartForm, a new RNI Workspace has been created as a follow-on to the original study. You can use the breadcrumb trail at the top of the page to navigate back to the Study Workspace.
Submit RNI
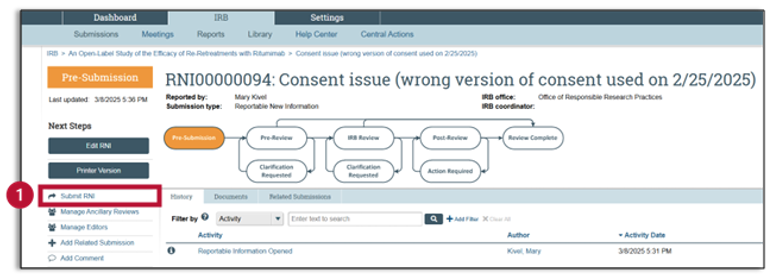
- After exiting the RNI SmartForm, you are now in the RNI Workspace. Your RNI is completed, but not yet submitted. You will notice that the current status is “Pre-Submission.” Click on “Submit RNI” in the left navigator, and a pop-up window will appear.
- You will now be back in the study workspace. The RNI submission is complete but has not yet been sent to the IRB. If you look at the workflow map, you can see that the RNI is still in pre-submission.
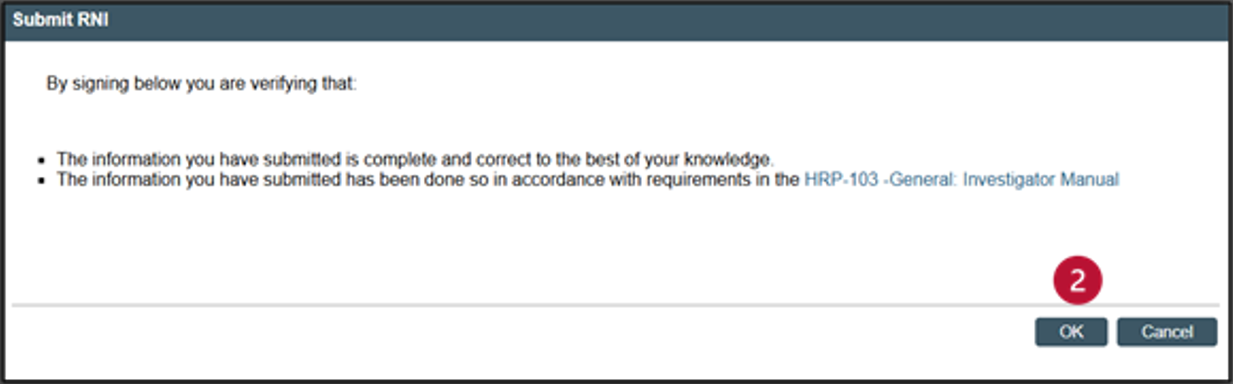
- After you submit your RNI, you will see that your RNI submission has now moved to “Pre-Review.”