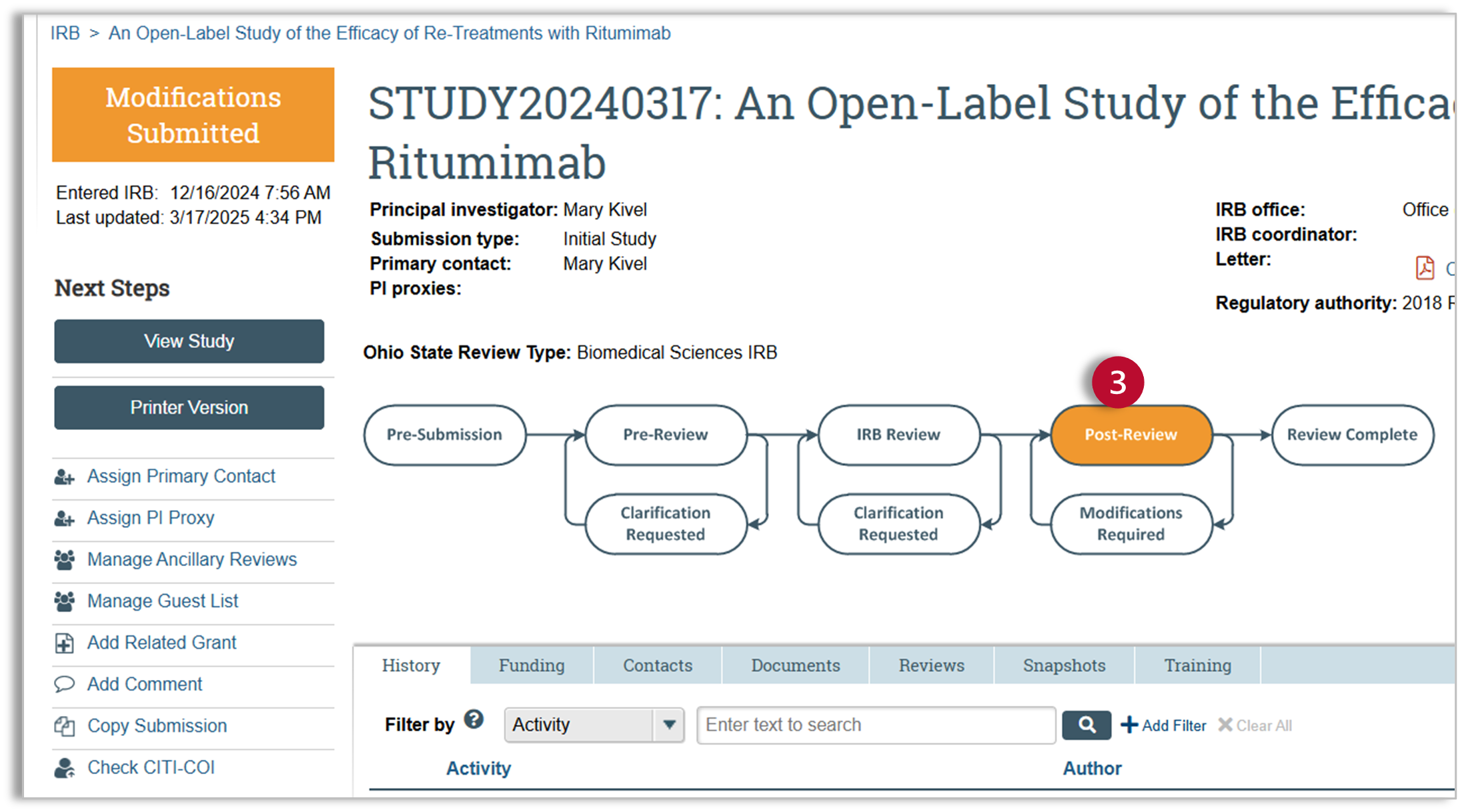
Huron IRB - Respond to Modifications Required
Estimated Reading Time: 3 MinutesAfter the study has been reviewed by the IRB, the IRB may have made the determination that modifications are required before a final approval letter can be sent.
Determination Letter
The PI will receive a determination letter, letting them know that a modification is required. The determination letter will indicate:
- Category: The letter will indicate the category of modification. There are three modification categories:
- Modifications Required to Secure “Approved”
- Modifications Required to Secure “Not Human Research”
- Modifications Required to Secure “Human Research, Not Engaged”
- List of required modifications: The letter will detail the required modifications, which will be bolded on the determination letter.
- Required submissions: The determination letter will provide a list of required submissions. For most modifications, ORRP will need both a clean copy of any modified document as well as document detailing the tracked changes
- “Clean” Copy: The PI or study team should submit a clean copy of the modified document. Use the “Update” button next to the original document to add the clean version of the document.
- Tracked Changes: The PI or study team should submit a tracked changes version of the modified document unless the document is in Word. The Huron system has the ability to compare tracked changes for Word documents, but does not have this ability for PDF, Excel, or other document types. If your changes are in any format other than Word, you will need to use the “Add” button to add a tracked changes document.
- Timeline: The letter will provide a response timeline. In this example, the letter indicates that the study team should respond within two weeks of receipt of the letter.

Navigation
When you have completed the necessary modifications, navigate to your study.
- You will find your study on the IRB tab
- Select “Submissions” in the sub-navigator to see your IRB submissions.
- Your study will be on the “In Review” tab
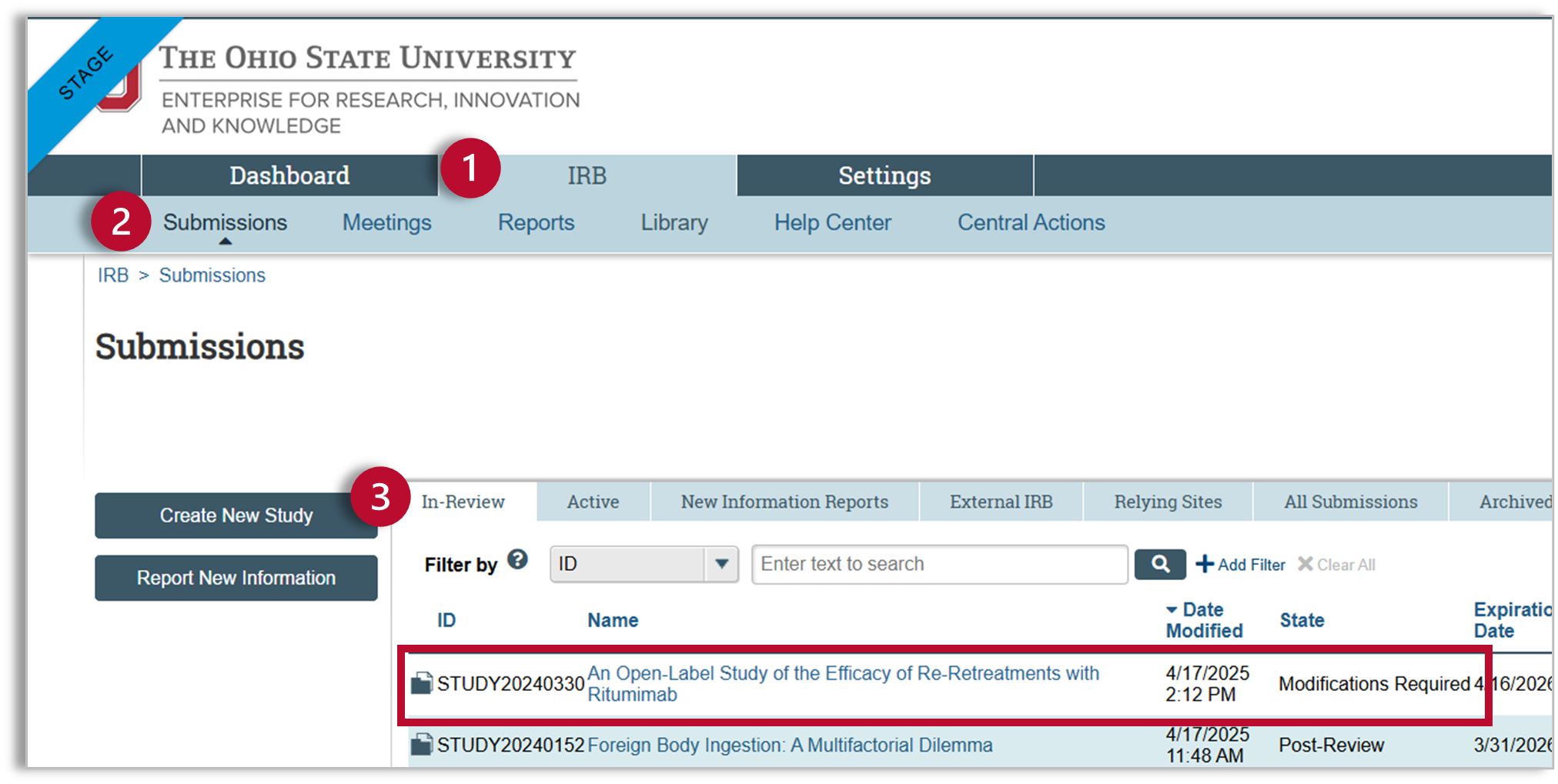
Study Workspace
- You will see the Determination Letter in the study history. You can click on the letter for more details.
- Editing is unlocked when the study is in “Modifications Required.” Click on the “Edit Study” button to open the SmartForm and make your required changes.
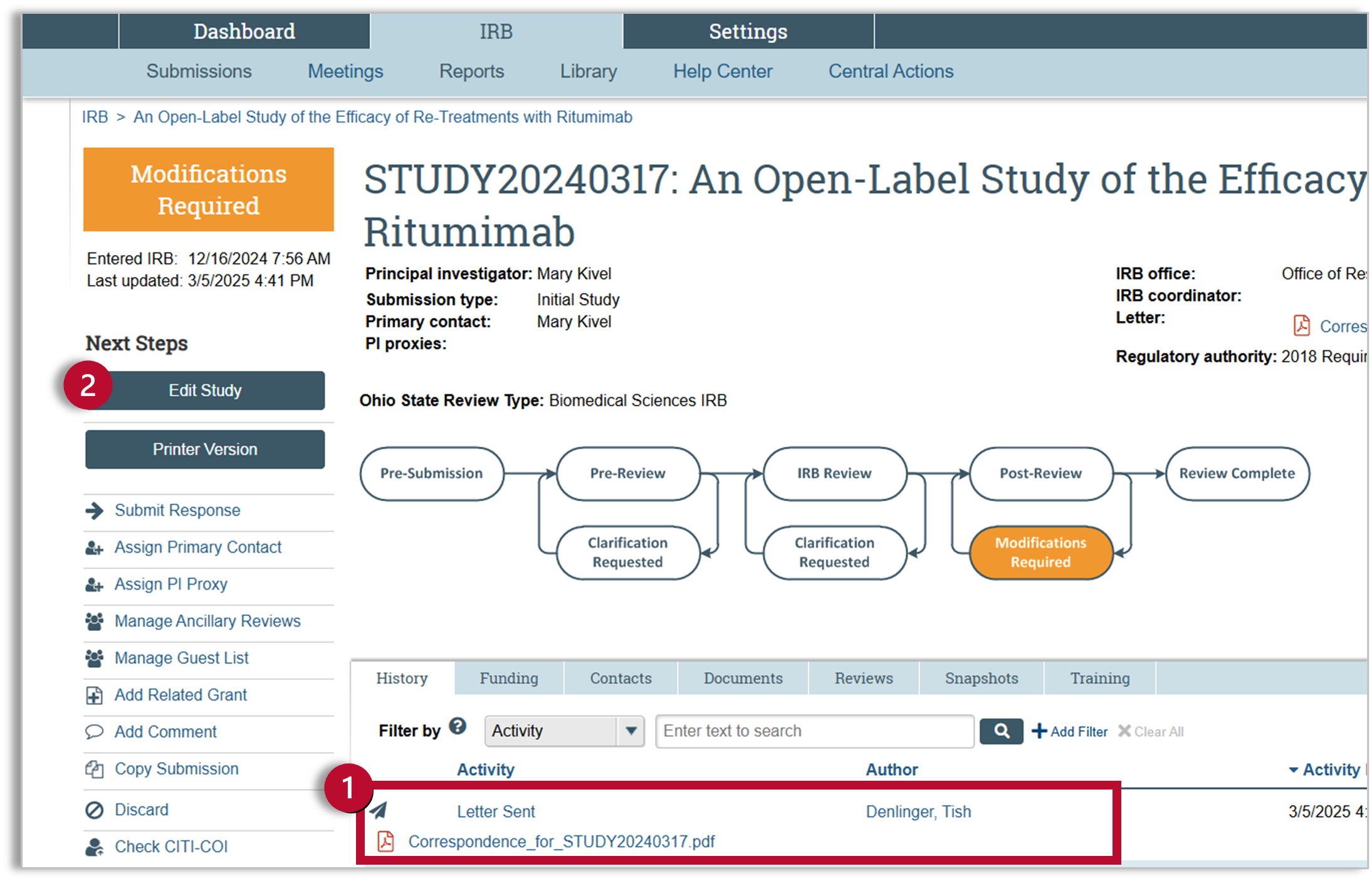
SmartForm
Edit the SmartForm
Navigate to the area of the SmartForm relevant to your required changes. Make the requested changes.
Adding Required Documents
Any time you make a change in the SmartForm, you can use the compare feature to compare changes.
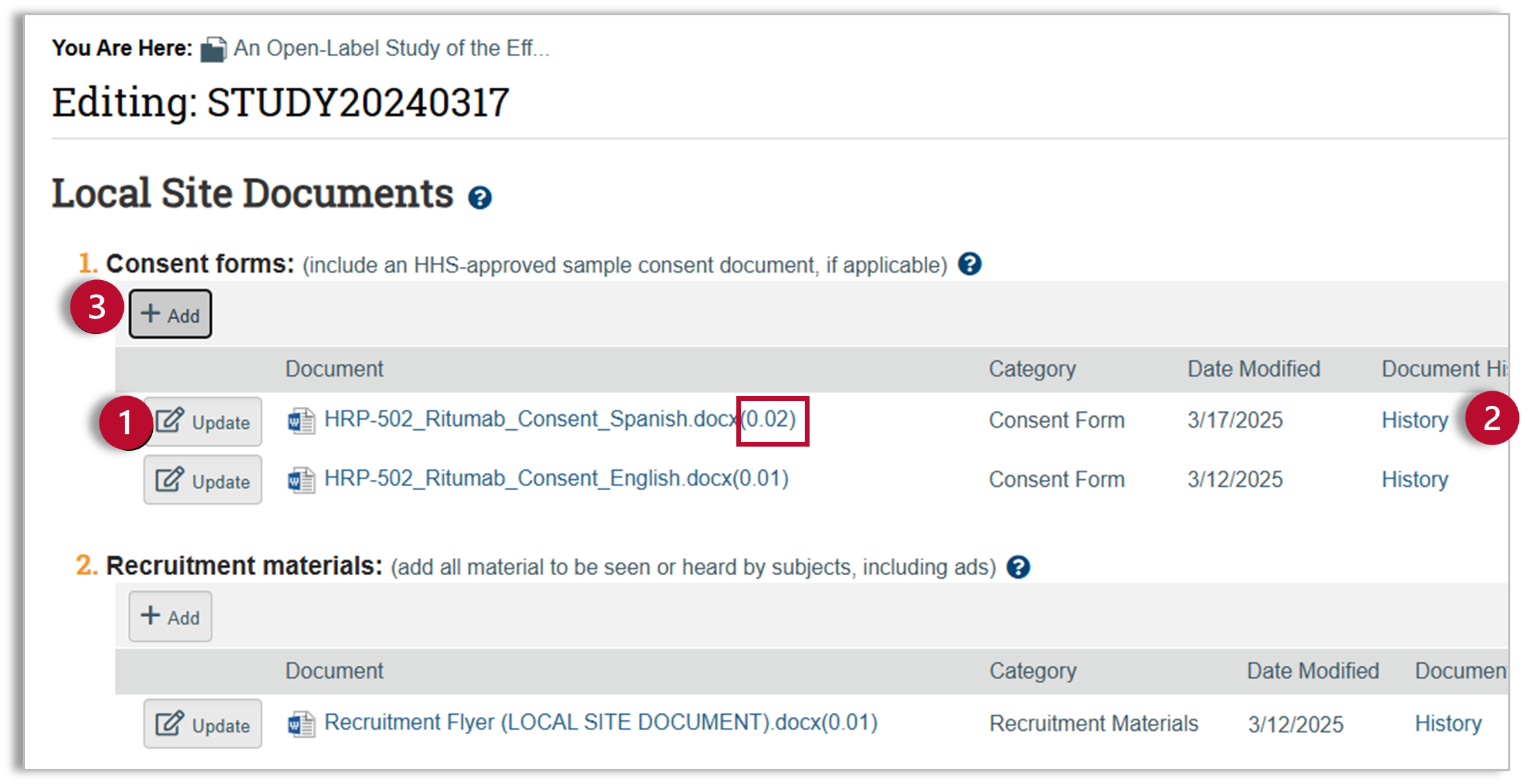
- Clean Copy: Submit a clean copy of any modified documents using the “Update” button. After you upload a new copy of the document, the decimal following the document title will increase indicating multiple versions of the document.
- Tracked Changes- Word documents: For Word documents with multiple versions (indicated by an increased decimal following the document title), you can click on “History” to view tracked changes. You do not need to upload a separate tracked changes document for Word files.
- Tracked Changes- Other File Types: For updates to files in formats other than Word, use the “Add” button to add a separate Tracked Changes version of the document.
Submit Clarification Request
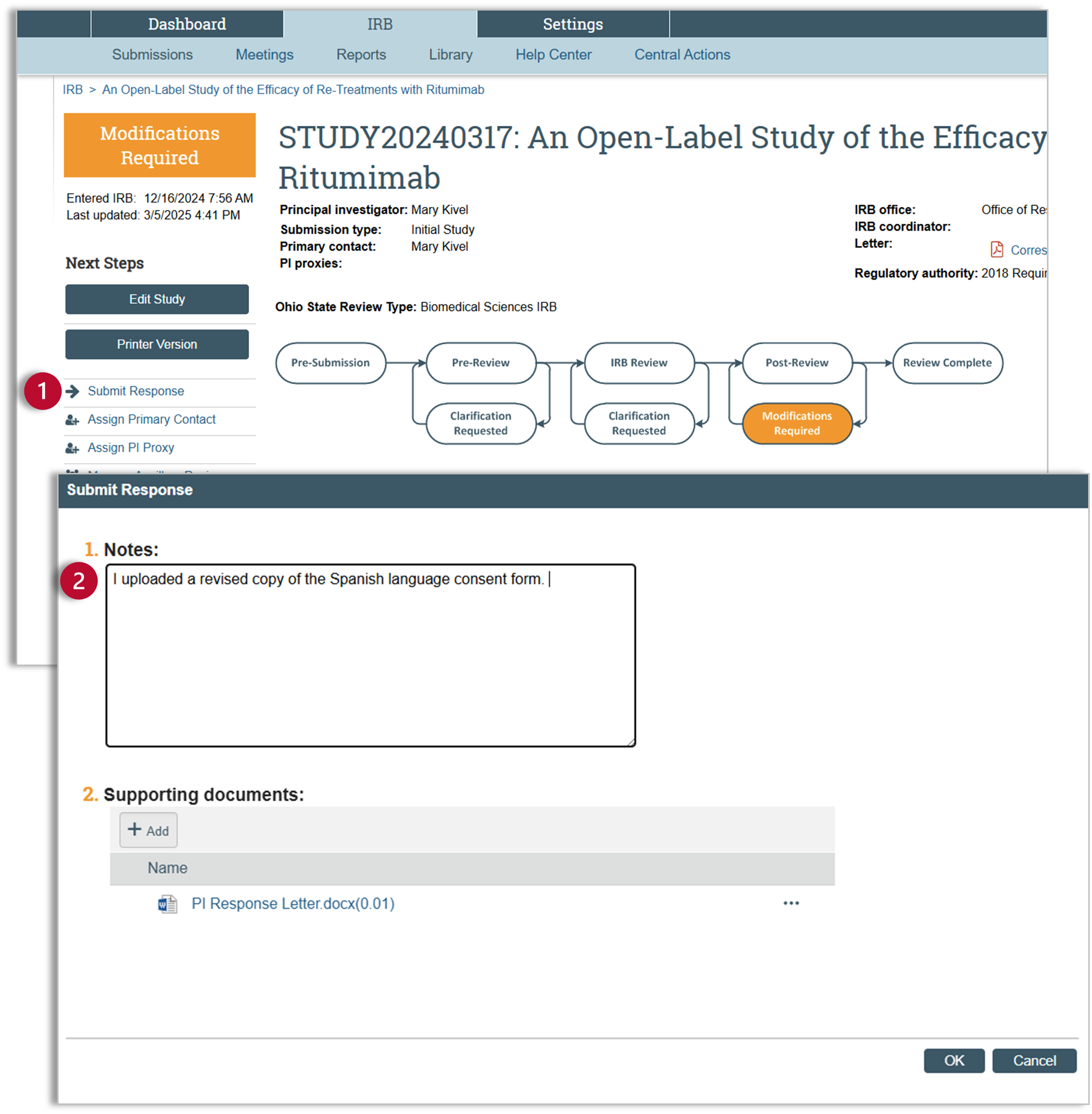
- After making the required changes and exiting the SmartForm, you will be back in the study workspace. You will complete the clarification request by submitting the response. Click on “Submit Response” and a pop-up window will appear.
- Use the “Submit Response” pop-up window to detail your changes. If your Determination Letter asked for a point-by-point response, attach your PI Response Letter detailing those changes. Once you have described your changes or uploaded your document, click “OK".
- After submitting your response, you will notice that the status of the submission is now “Modifications Submitted” and the workflow map has moved back to “Post-Review.” ORRP staff will review your modifications and finalize your documents.