Huron IRB - Create and Submit a Continuing Review
Estimated Reading Time: 4 MinutesPrior to Submitting in Huron
Prior to submitting your Continuing Review in Huron, you will first need to complete the HRP-215-Template: Continuing Review Supplement Form. This editable Word document can be found in Huron Library [IRB > Library > Templates]. This form is required for all Continuing Reviews.
Navigation
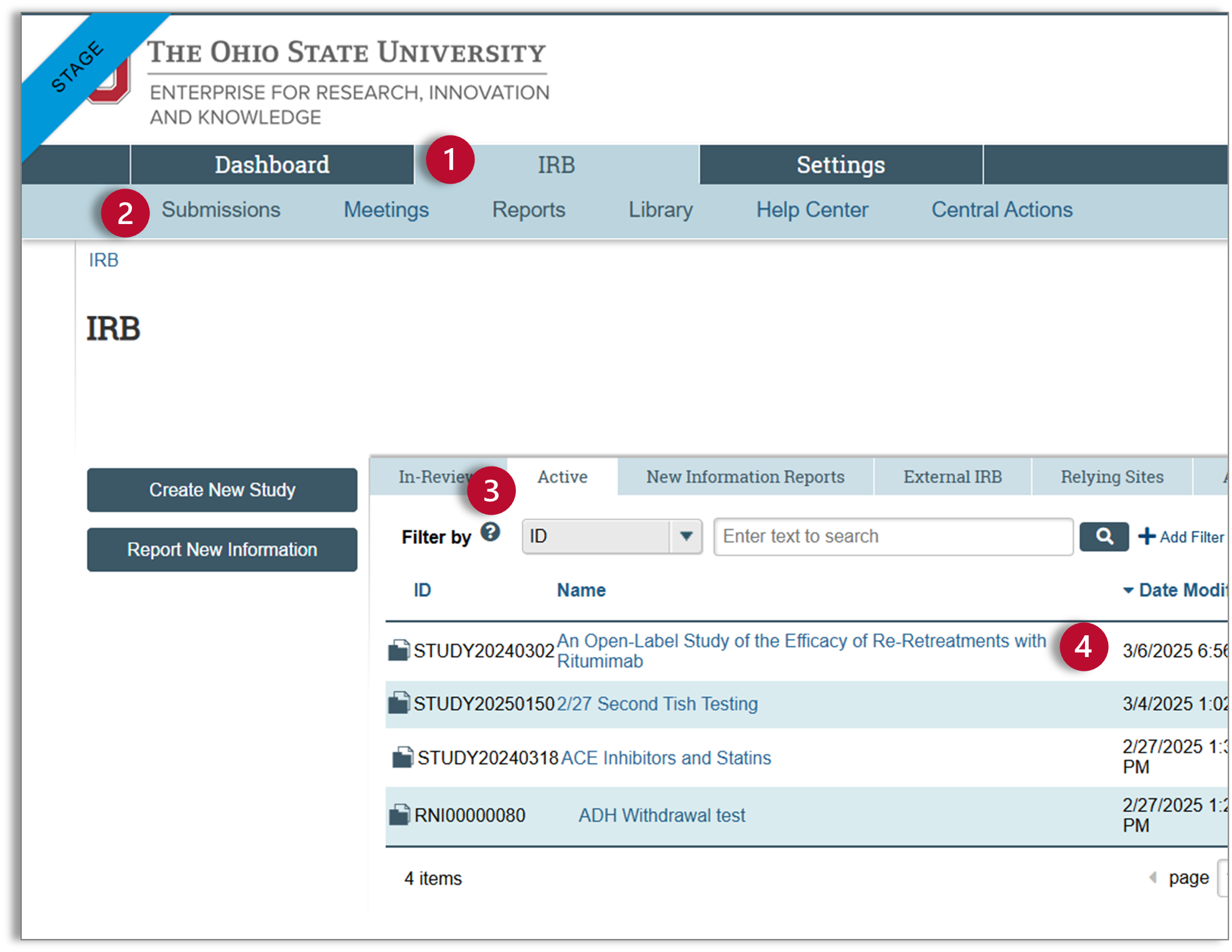
- Make sure you are on the IRB tab.
- Select “Submissions” in the sub-navigator to see your IRB submissions.
- Select the “Active” tab.
- Find and select the study related to your Continuing Review.
Study Workspace
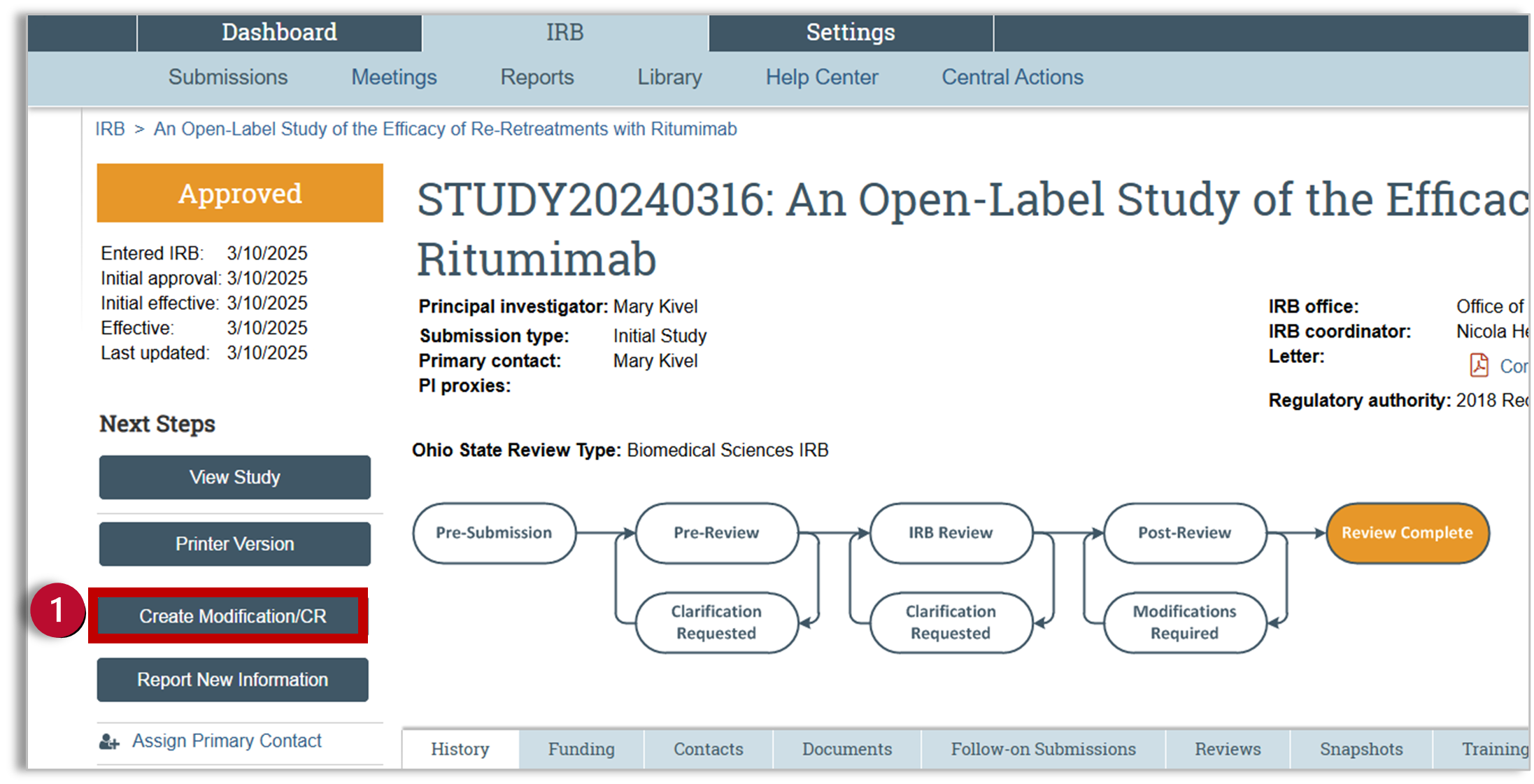
- Once inside the study workspace, you will select “Create Modifications/CR” to open the Modifications and Continuing Review SmartForm.
Continuing Review SmartForm
Selection Page
- Identify the purpose of the submission
- Continuing Review: Select Continuing Review if you want to extend the approval period or close the study.
- Modification/Update: Select Modification/Update to make a change to the study. Refer to the “Create and Submit a Modification” article.
- Modification and Continuing Review. Combined Modifications and Continuing Reviews are limited to changes involving study team updates. More significant modifications should be submitted separately.
- Click Continue. The SmartForm will vary depending on your selection. This sheet will provide information for Continuing Review.
Continuing Review/ Study Closure Information Page
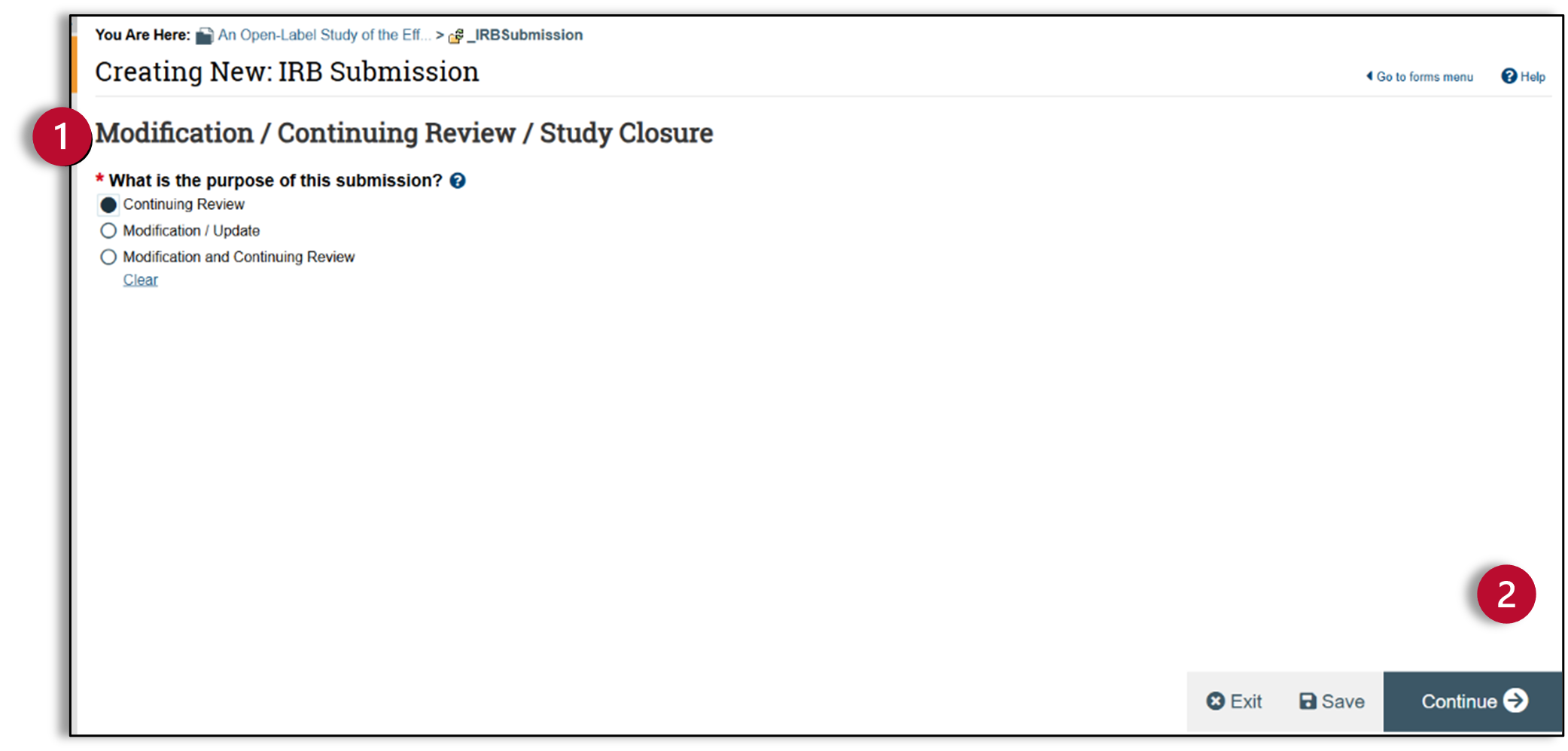
- Specify enrollment totals at this investigator’s sites: Specify enrollment totals for all sites at which the sIRB PI is responsible for the research, including all Local Research Locations identified for the study. This does not include participating sites.
- Specify enrollment totals at this investigator’s sites since last approval: Enter any new enrollments at all Local Research Locations.
- Specify enrollment totals study-wide: Include all sites that are conducting this protocol, including Local Research Locations and participating sites.
- Research Milestones: Read these six statements carefully, because some of the options contain two different statements separated by "OR". If either statement is true, check the box. If the first four research milestones above are complete, the study will be closed to discontinue IRB oversight.
- Do any investigators or research staff have a financial interest related to the research that was not described in a previous application?
If an individual has a new financial interest, provide a copy of the Conflict-of-Interest Committee's determination regarding the interest. Attach a copy using the Supporting Documents page, which appears later in the submission forms. - Check the items that are true since the last IRB approval for all sites involved in the study: Read the statements carefully and put a check next to all items that are true since the last IRB approval for all sites involved in the study. Items left unchecked, will require an explanation or action as directed in the HRP-215: Continuing Review Supplemental Form.
- Attach supporting documents. Attach all supporting documents, making sure to include:
- HRP-215: Continuing Review Supplemental Form, including a summary of research progress and explanation of each item left unchecked for question six.
- Sponsor's progress report or annual report, if available.

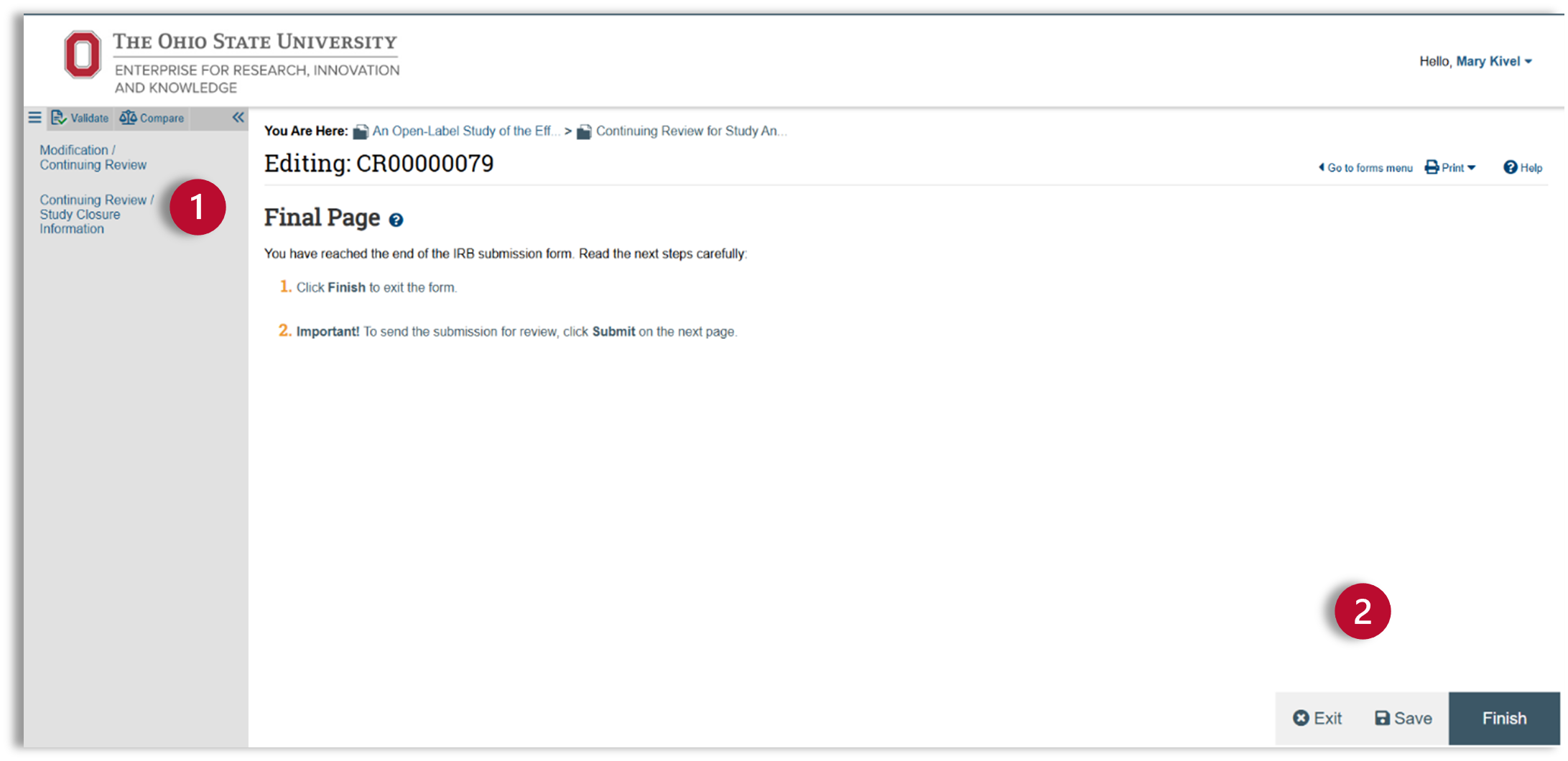
Final Page
- After you have completed the Continuing Review /Study Closure SmartForm, you will come to the Final Page. You can check your submission for errors using the validate and compare features at the top of the left navigator.
- After you have corrected any errors, click finish and exit the SmartForm.
Continuing Review Workspace
Before entering the Continuing Review, you were in the Study Workspace. After completing the Continuing Review SmartForm, a new Continuing Review workspace has been created as a follow-on to the original study. You can use the breadcrumb trail at the top of the page to navigate back to the Study Workspace.
Submit Continuing Review
- After exiting the Continuing Review SmartForm, you are now in the Continuing Review Workspace. Your Continuing Review is completed, but not yet submitted. You will notice that the current status is “Pre-Submission.” Click on “Submit” in the left navigator, and a pop-up window will appear.
- Read the information in the pop-up window and click “OK” if you agree.

- Your continuing review has now been submitted. You will notice that the status is now “Pre-Review.”
