Huron IRB - Create and Submit a New Study
Estimated Reading Time: 11 MinutesPrior to the Study
Studies can be entered by either the Principal Investigator (PI) or the Study Team. However, only the PI can submit the study. Before you enter the study in Huron, you should first complete the appropriate protocol template for your category of study.
The biomedical and cancer, behavioral and social sciences, exempt, and secondary research protocol templates must be used for all investigator-initiated research studies written and designed by an Ohio State investigator
- HRP-503- Template- Protocol – Biomedical and Cancer Research
- HRP-503a- Template- Protocol – Behavioral and Social Sciences Research
- HRP-503b- Template- Protocol – Secondary Research
- HRP-503c- Template- Protocol – Exempt Research
For multi-site research studies not written or designed by an Ohio State investigator (i.e., provided by an external site or sponsor), the Ohio State or External Site supplemental protocol templates must be completed and uploaded with the external protocol.
- HRP-504- Template- Protocol – External IRB Site Supplement
- HRP-505- Template- Protocol – Ohio State IRB Site Supplement
This editable Word document can be found in Huron Library [IRB > Library > Templates].
Navigation
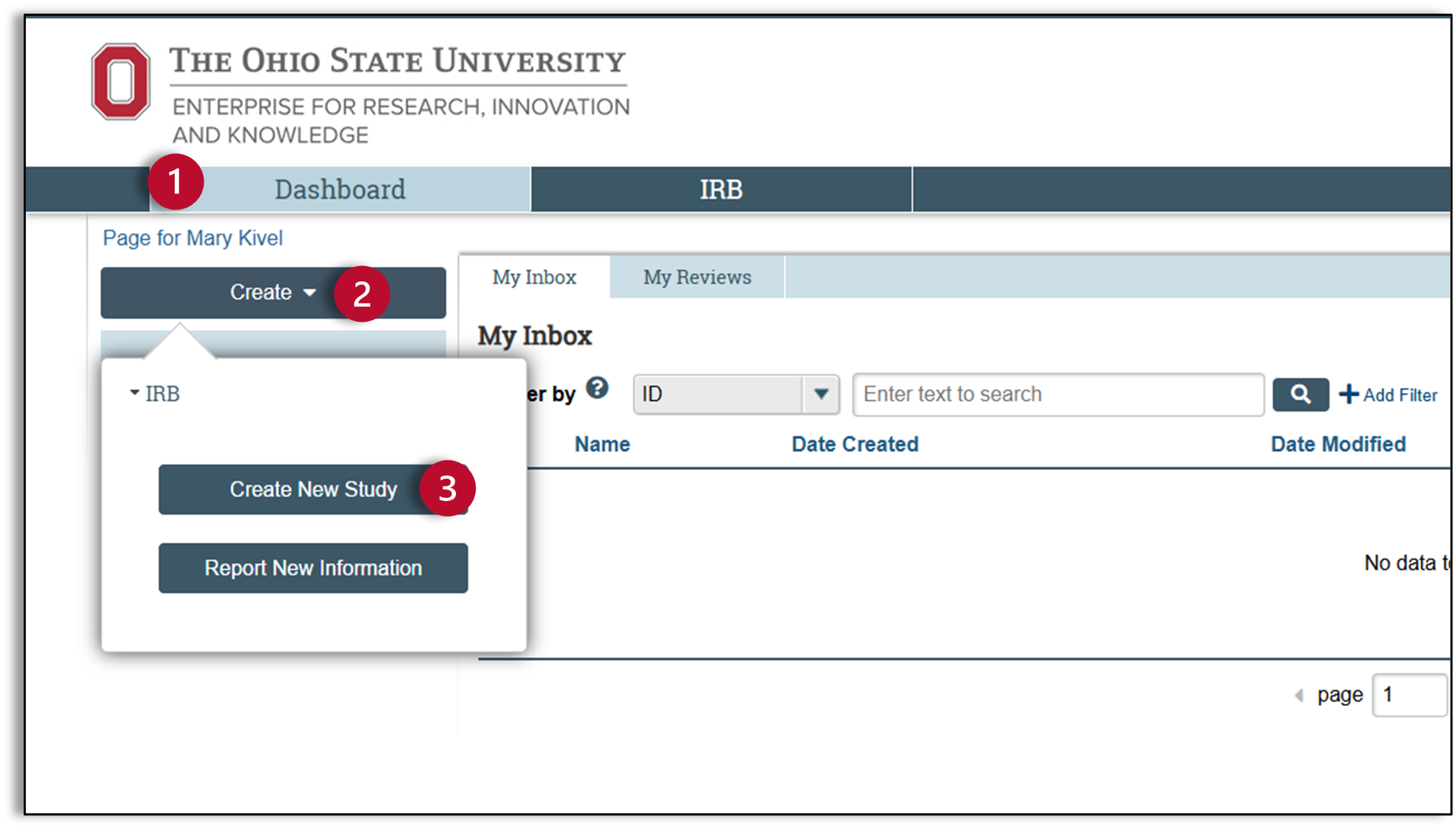
- Make sure you are on the Dashboard
- Click Create
- A pop-up window will appear. Click Create New Study.
SmartForm
Basic Study Information
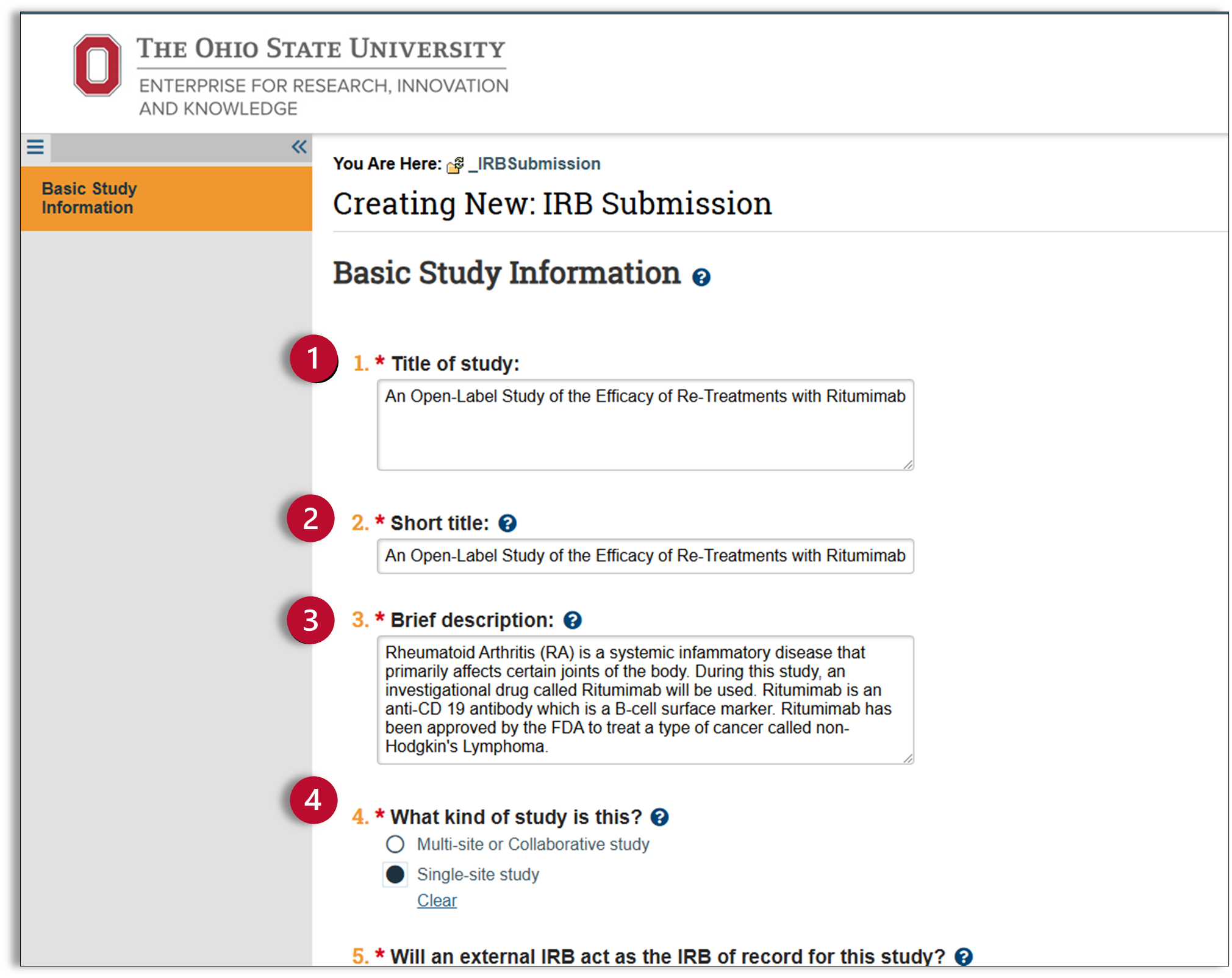
- Title of study: Enter your study title, this can be copied and pasted directly from your protocol document.
- Short title: The short title is used to identify your study throughout the Huron system and is also the title that is used on participant documents, such as the consent form. The “Short Title” and the “Title of the Study” (1) should match, unless your title is longer than 277 characters. If your title is longer than 277 characters, you should shorten the title but try to remain as close to the original title as possible.
- Brief description: Enter a brief description of your research. This can be copied and pasted directly from your protocol document.
- What kind of study is this? Identify whether your study is a multi-site or single-site study.
- Multi-site or Collaborative study: Multiple institutions are involved in this study.
- Single-site study: Ohio State is the only IRB of record on this submission.
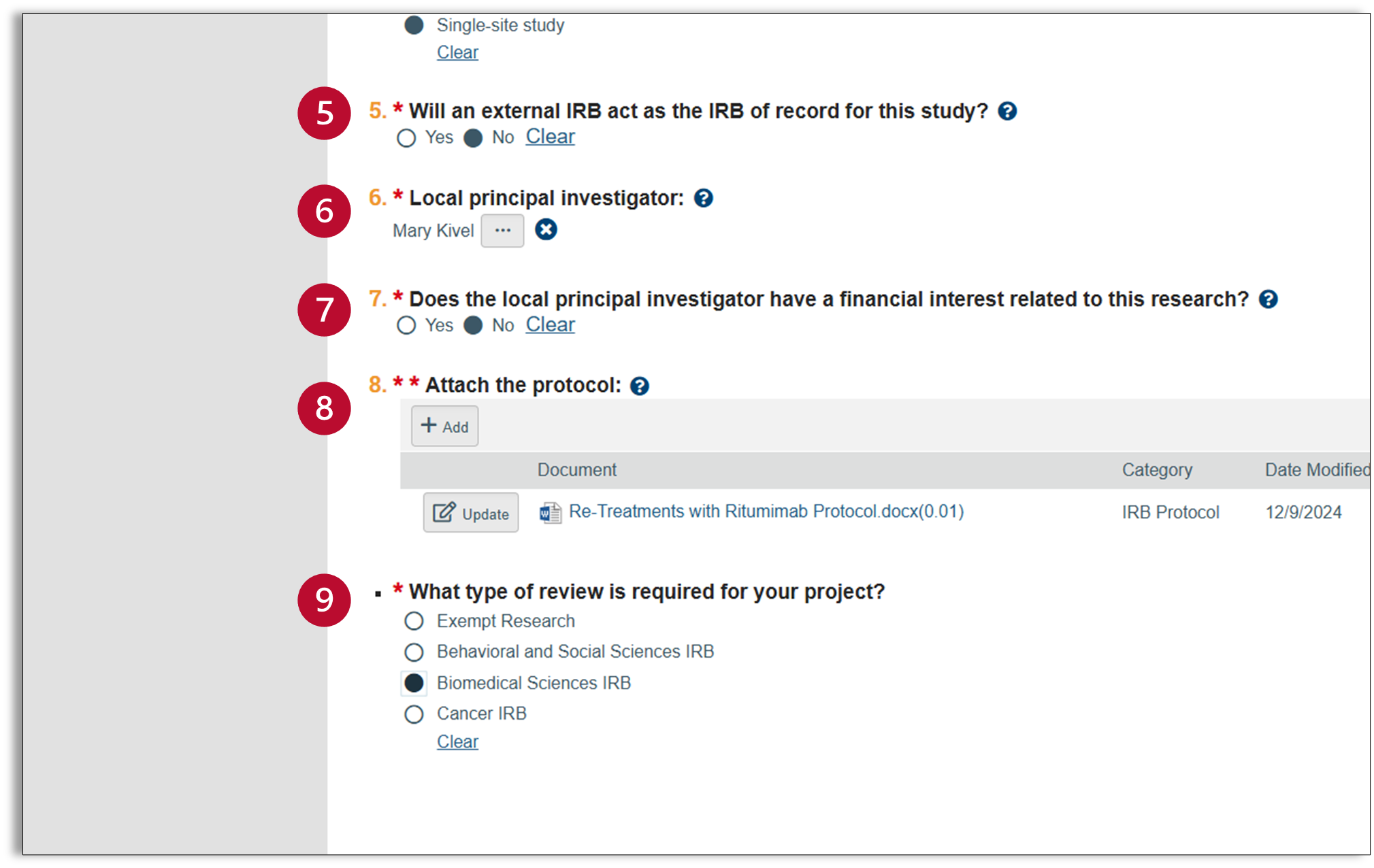
- Will an external IRB act as the IRB of record for this study? If another institution will be the IRB for your study, select “yes.” If Ohio State will serve as the IRB for your study, select “No.”
- If you select “No,” an additional question should appear at the bottom of your form (9) asking you to identify the type of review required for your study.
- Local principal investigator: This will default to the name of the person entering the study. If you are not the PI for this study, click on the ellipses and a full list of Ohio State investigators will appear. Select the PI from this list. If the PI is not listed, please contact ORRP.
- Financial Interest: The PI is required to disclose and conflicts of interest related to this study. For questions related to conflicts of interest, please contact the Conflict-of-Interest team at conflictinfo@osu.edu.
- Attach Protocol: Use the “Add +” button to upload your protocol document. A pop-up window will appear and allow you to upload your document. You also have the option to include an alternate file name and version number.
- What type of review is required for your project? This question will only appear if you selected “No” for question 5. Select the appropriate choice for your study.
- Exempt Research: this category is for research that is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories in the federal IRB regulations.
- Behavioral and Social Sciences IRB: reviews research originating from a variety of disciplines; however, does not review FDA-regulated research or research otherwise involving medical procedures, drugs, or devices.
- Biomedical Sciences IRB: reviews biomedical research, excluding cancer. research.
- Cancer IRB: reviews all aspects of observational and interventional cancer research
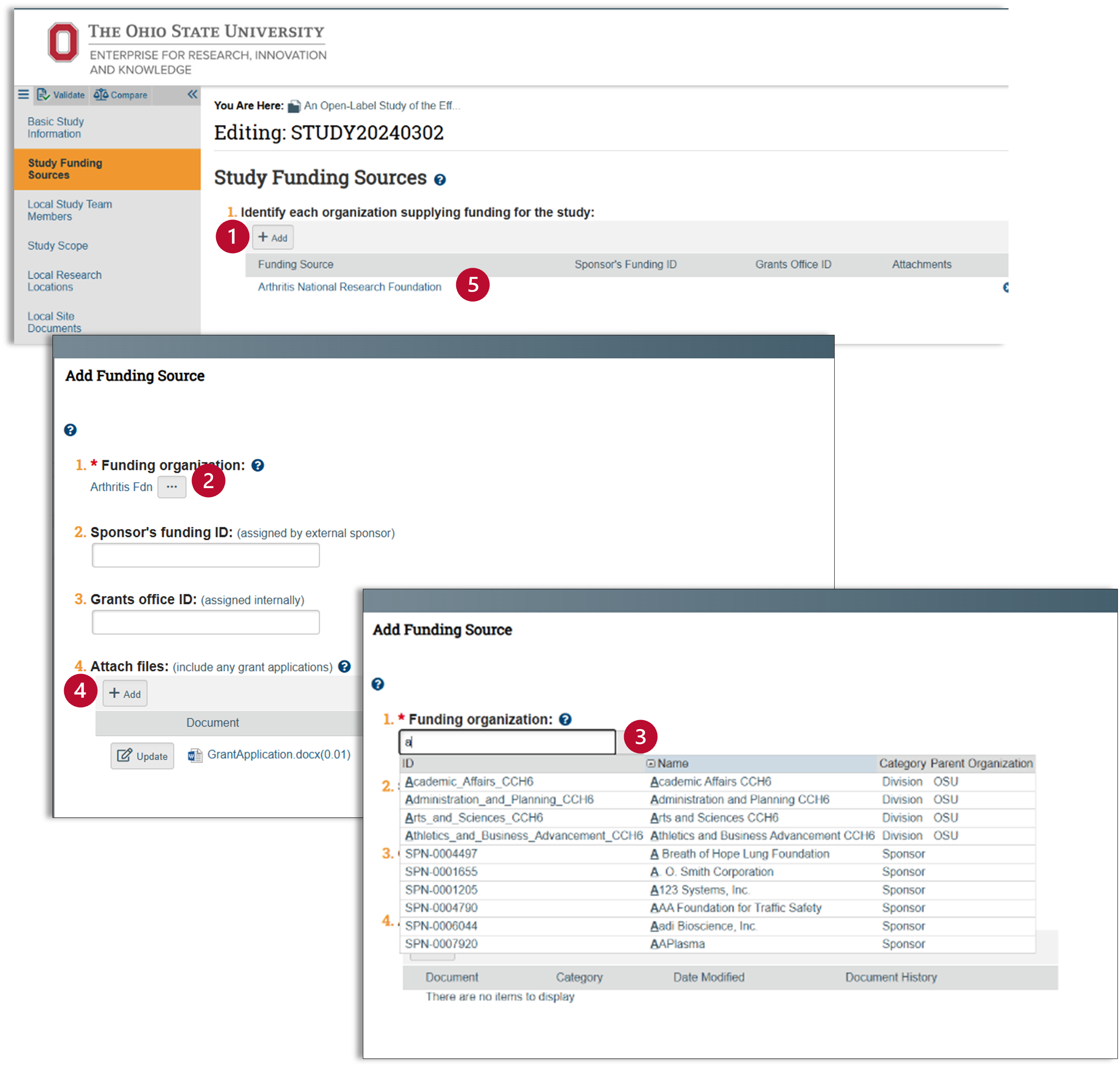
Study Funding Sources
- Click on the “+Add” button to add funding sources for the study.
- A pop-up screen will appear. Use this tab to enter both internal and external funding sources. Click on the ellipses […] to access an extensive list of over 13,000 funding sources
- Use the second pop-up to add your funding sources.
- Use the wild card “%” to search for your funding source.
- For all award internal to OSU, use “SPN-0006020 Ohio State University Internal Award”
- Upload a grant application for external funding sources.
- Check to make sure your funding source is correct.
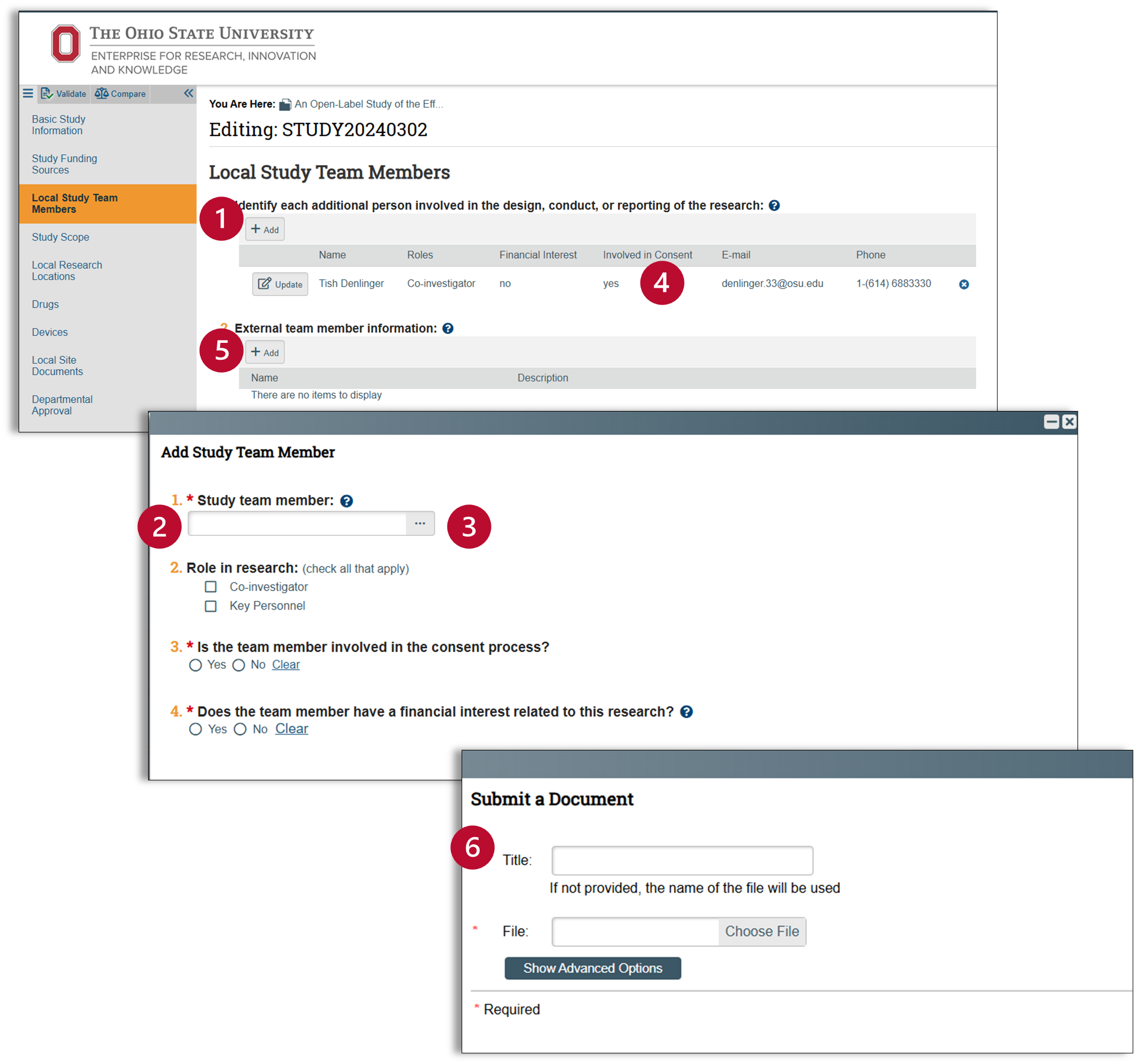
Local Study Team Members
Add study team members
- Click on the “+Add” button after the statement “Identify each additional person involved in the design, conduct, or reporting of the research.”
- A pop-up window will appear. For each team member, indicate their role in the research, the consent process, and disclose any financial interests related to the research. Once you have completed all fields for your first team member, you can select "OK and Add Another" to continue adding the rest of your study team.
- The ellipses […] will allow you to access a list of Ohio State affiliated individuals. The help text provides additional information for external members. Collaborating individuals must have an active Ohio State ID and complete required training. There in an Ohio State job aid for creating a guest account. This step will need to be completed for external members before they can be added in Huron. In addition to an Ohio State ID, a CV should also be added for any external members (step 6).
- After you have added all of your study team members, click okay and then check to make sure that they are listed correctly.
- There is an additional entry field for external members that will allow you to upload the CV for an external member. Click the “+Add” button.
- Use the pop-up window to add a CV for external members.
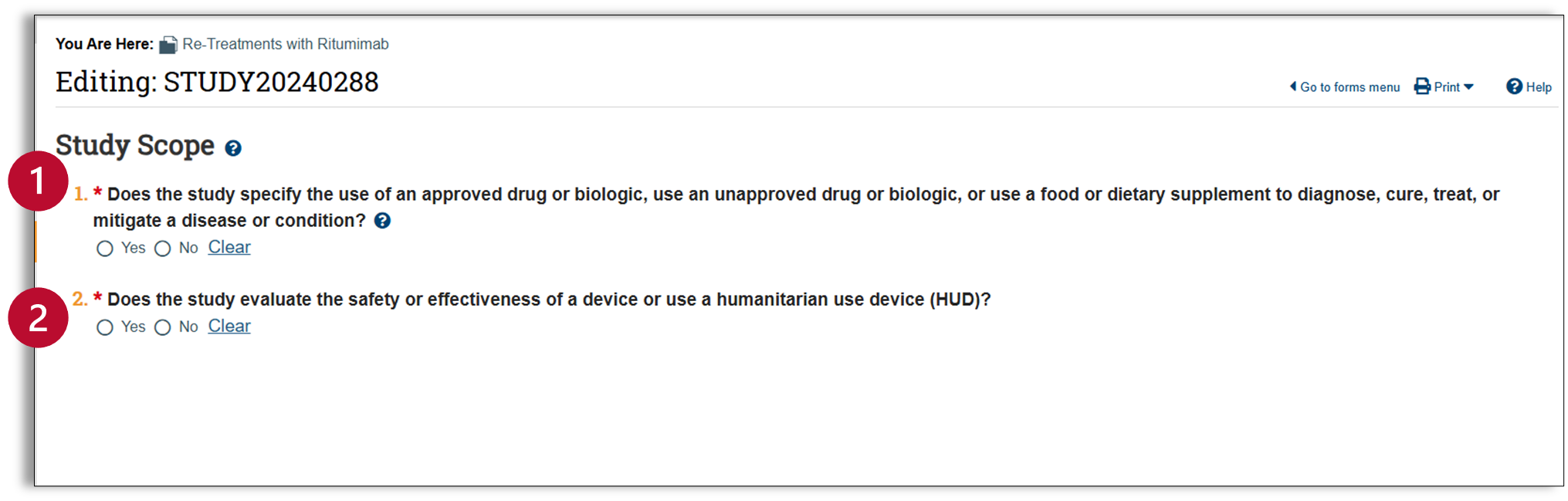
Study Scope
- Drugs and biologics: This first question asks “Does the study specify the use of an approved drug or biologic, use of an unapproved drug or biologic, or use a food or dietary supplement to diagnose, cure, treat, or mitigate a disease or condition? If the protocol requires one or more subjects to use the drug, biologic, dietary supplement, or food as part of study participation, you should answer yes to this question. This will add an additional tab titled “drugs” in the left navigator.
- Devices: This second question asks “Does the study evaluate the effectiveness of a device or use of a humanitarian use device (HUD)?” If you are unsure if your device qualifies as a HUD, please reach out to IRBinfo@osu.edu. Answering yes to this question will add an additional tab titled “devices” in the left navigator.
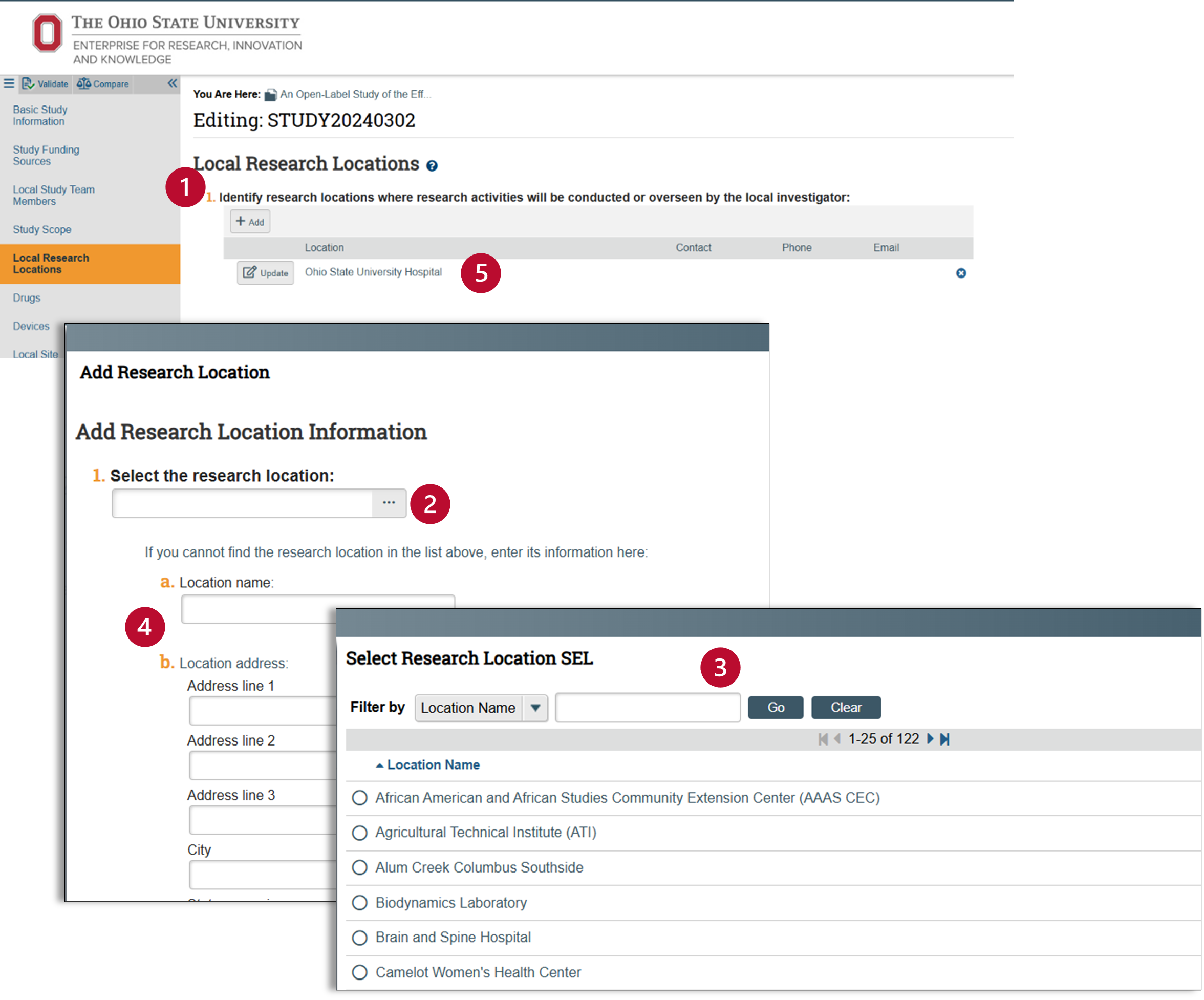
Local Research Locations
This question asks you to identify research locations where research activities will be conducted or overseen by the local investigator.
- Click on the “+Add” button and the “Add Research Location” pop-up window will appear.
- Use the ellipses […} to access a list of local research locations.
- Use the selection field to identify your location. Use the wild card “%” to search for your site.
- If your study involves a medical facility, please search for the specific location.
- For non-medical sites on the OSU campus, please use "Ohio State Columbus Campus."
- You should also include any other local, off-campus sites, such as a local elementary school, nursing home, or a private physician's office.
- If you have a multi-site study, you do NOT need to list the participating sites, because those sites would rely on the reviewing IRB.
- If you cannot find your local research location in the research location list, manually enter the location.
- Check to make sure your location is correct.
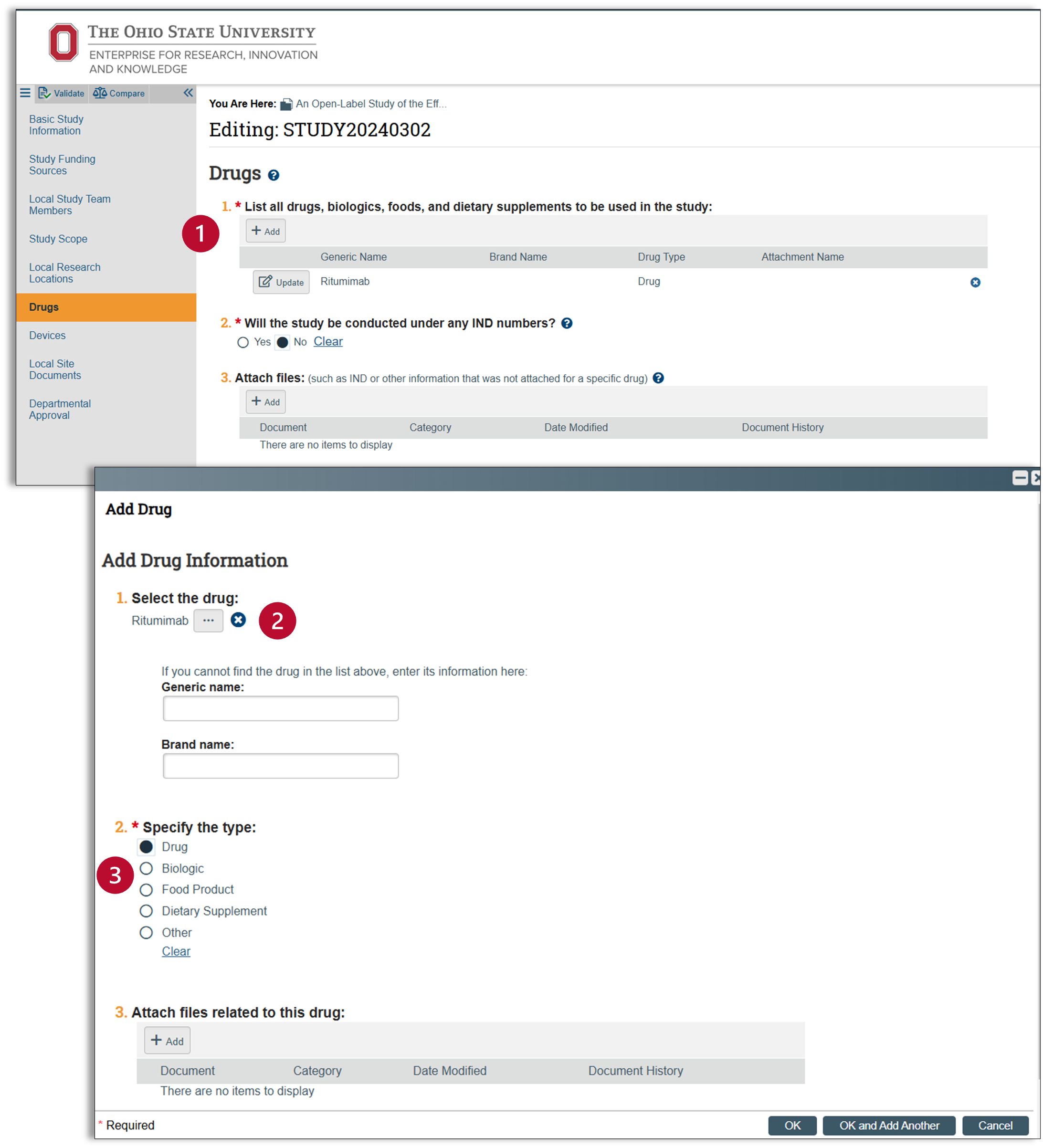
Drugs
This page will only appear if you answered "yes" to the first question on the study scope page.
- Click on the add button for a pop-up window that will allow you to enter in any drug, biologic, food product, or dietary supplements used in the study.
- Use the responsive text to identify the items listed in your protocol. Huron maintains a robust list in this category, however if your item is not on this list, please enter it on the line provided.
- Next, specify your item type, and attach any related files. Click okay and then look to make sure your entry is correct.
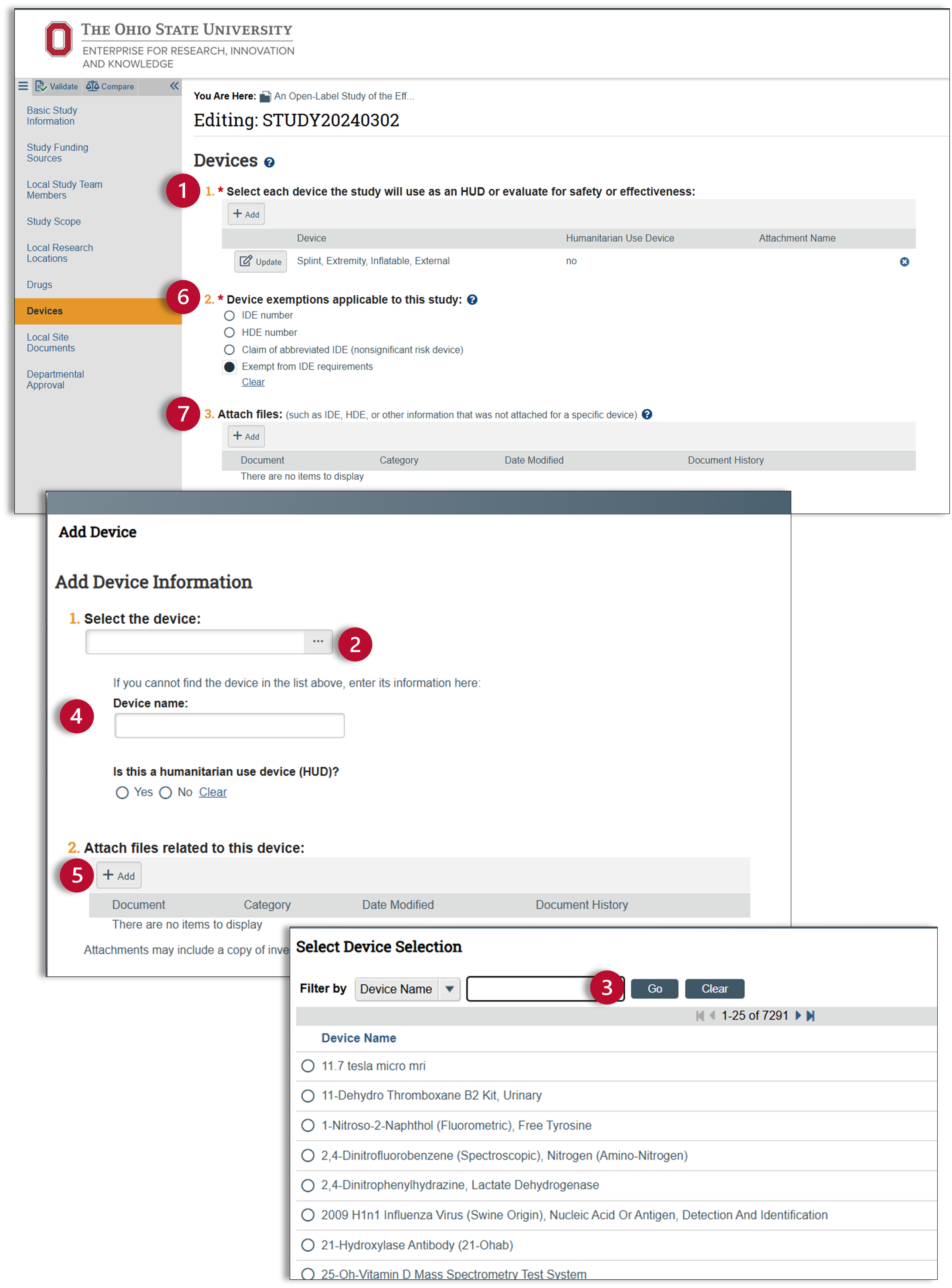
Devices
The devices tab will only appear if you answered “yes” to question two on the study scope page.
- Click on the add button for a pop-up window that will allow you to enter in your device information.
- Click on the ellipses […] to access an extensive device list.
- Use this list to locate your device. Use the wildcard, “%”, to easily find your device in this list.
- If your device was not on that list, manually enter in the name of your device and indicate whether it is a humanitarian use device.
- If you have any files relating to your device, enter them here. Click okay and check again to confirm that your device information is correct.
- Question two is a required field, asking you about possible device exemptions. Use the help text for additional clarification. If you still have a question pertaining to device exemptions, you can reach out to ORRP at IRBinfo@osu.edu.
- The final question on this page is optional and provides a space for you to attach any documents related to any device exemptions. This attachment field is for documents relating to any potential investigational device exemptions or any humanitarian device exemptions. Attachments for general device information should be added in the device pop-up window.
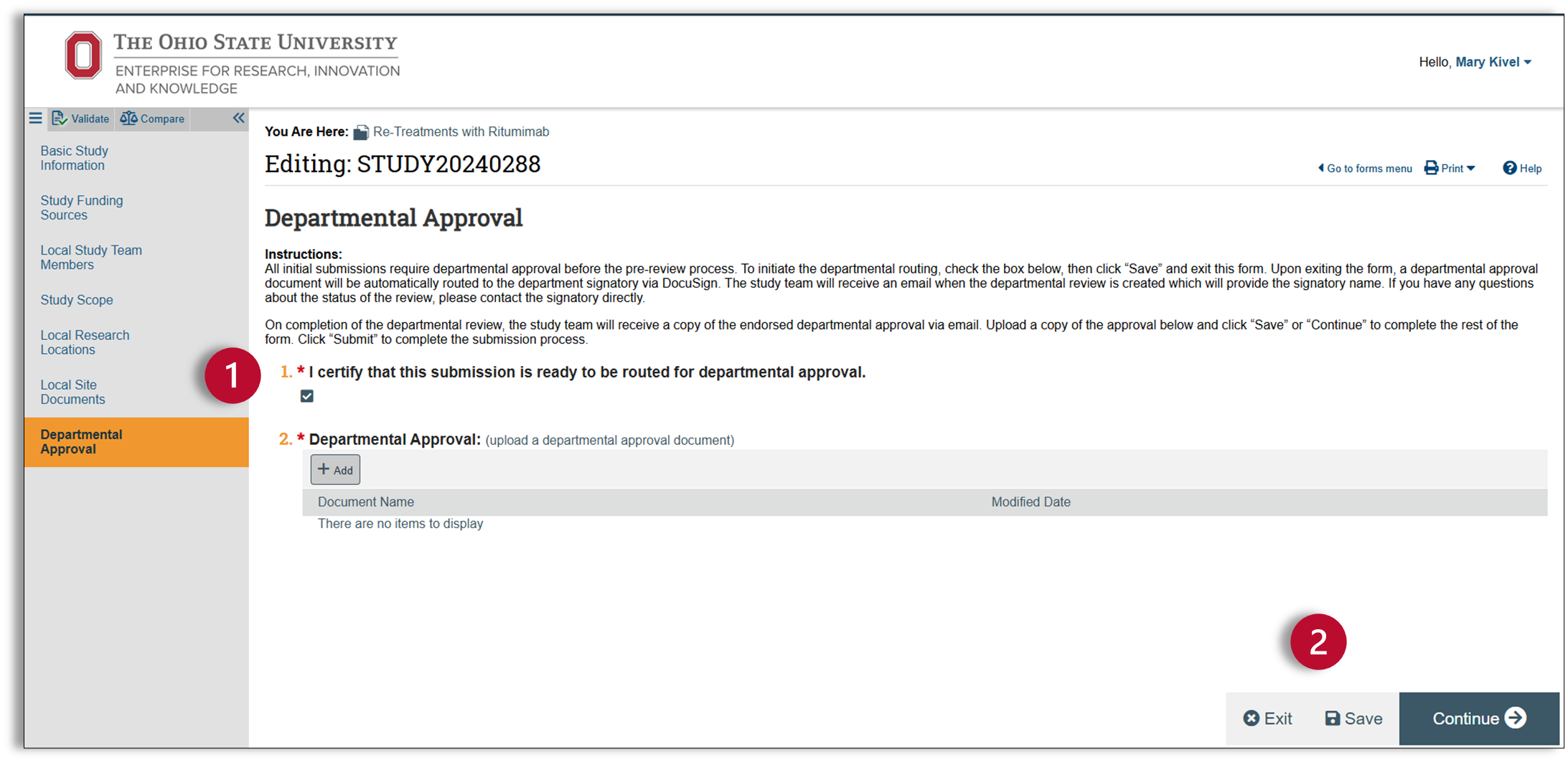
Department Approval
Ohio State requires depart mental endorsement of all regulated research protocols, including those for human subjects research.
- Once a new protocol is ready for review by the department endorser, check the box labeled ‘I certify that this submission is ready to be routed for depart mental approval.’ By checking this box and then clicking save, your protocol has now been routed for approval to your depart mental approver.
- You cannot proceed any further until you have the signed approval. The approval process happens outside the Huron system, so you can save and exit for now.
- The departmental approver will receive a notification email letting them know that a summary of the protocol information is ready for review in DocuSign. The PI will also receive a verification email letting them know that the protocol has been routed for departmental review. The approver will log into DocuSign to review and approve the protocol. Check with your department for their approval process and timelines. Your department approver may ask the research team for more information prior to endorsing the protocol.
- Once the departmental approver signs the protocol, the PI will receive a confirmation email from DocuSign, letting them know that the approval is complete. The PI can now download a P.D.F. copy of the signed approval from DocuSign.
- Once the PI has downloaded the departmental approval, they are now ready to submit. Log back into the Huron system. Once back in Huron, navigate to your dashboard, find your study, and click on edit study.
- Once you have opened the SmartForm, navigate to the Departmental Approval tab.
- Click on the “+Add” button to access a document submission window.
- Use the “Submit a Document” pop-up window to find and upload the Departmental Approval from your device.
- Check to make sure your approval has uploaded correctly and click continue.

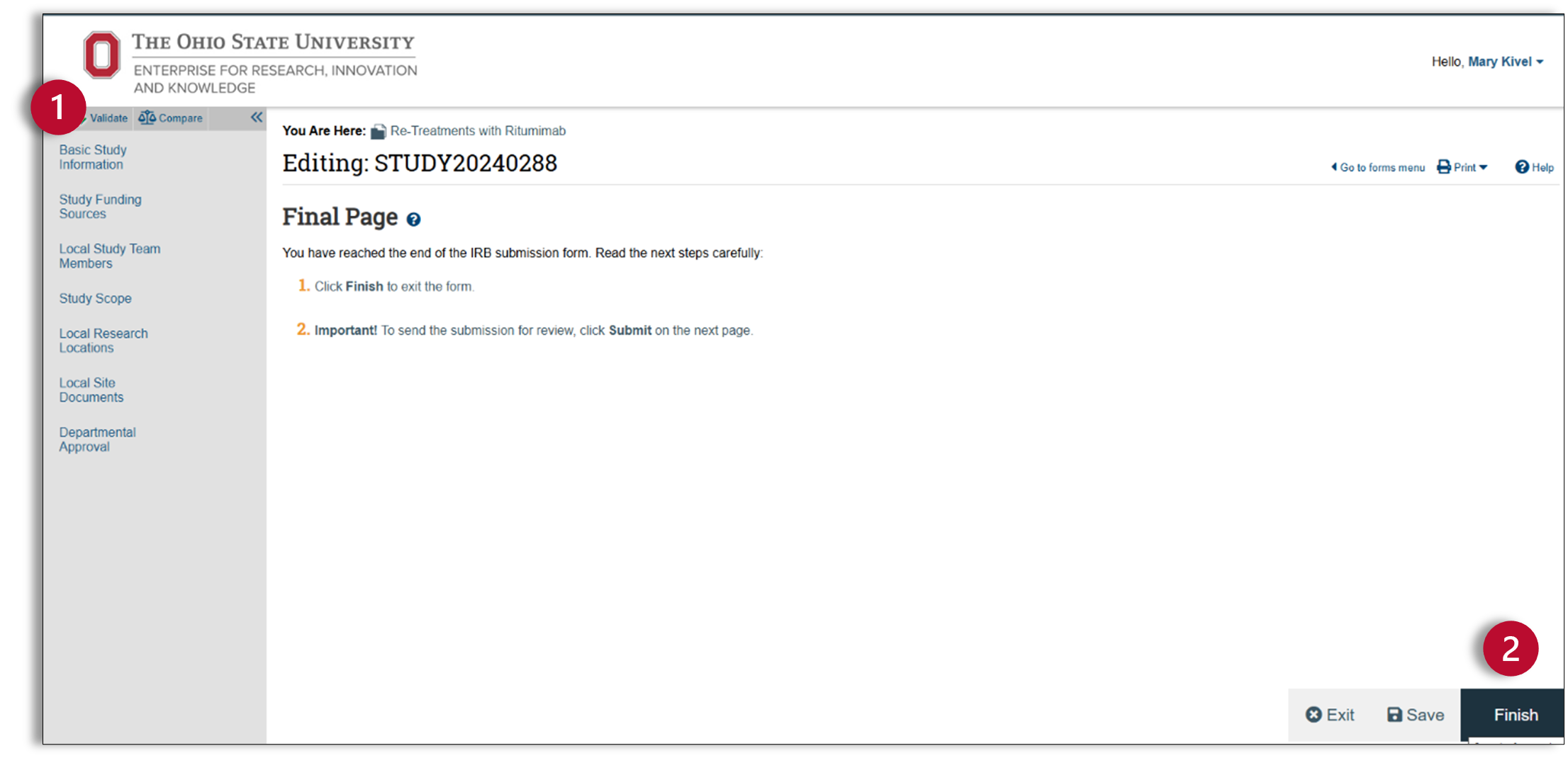
Finalize and Submit
- After you have clicked “Continue” on the Departmental Approval page, the final page of the Smart Form will appear. Before you finish the SmartForm, check your study for errors using the validate button at the top of the left navigator.
- After you have corrected any errors, you are now ready to finish the SmartForm. You will notice that the SmartForm now has a finish button. Click finish and exit the SmartForm.

- After exiting the SmartForm, you will now be back in the study workspace. The study is complete but has not yet been sent to the IRB. If you look at the workflow map, you can see that the study is still in pre-submission.
- The study staff can enter a study, but only the PI can submit a study. The PI will click “Submit.”
- After clicking “Submit,” an agreement page will appear. After reading this page, the PI or PI Proxy can click OK.

- The study is now submitted. Congratulations. You will notice the state has changed to pre-review on the study workspace, indicating that it is now In-Review.